Quantum mechanics isn’t just an abstract theory confined to textbooks and laboratories. It’s the very reason the vibrant colors on your TV screen pop, the tiny red light on your remote control works, and the laser in your grocery store checkout scanner reads a barcode in an instant. All of these everyday devices are powered by a beautiful and bizarre principle of physics: how atoms create light.
This post will guide you through the astonishing quantum physics behind two of our most common light sources: the humble LED and the powerful laser. We’ll show you how both work by a spectacular dance of energy inside atoms, proving that even the simplest light has a quantum secret.
The Atomic Light Switch: Understanding Energy Levels
To understand how lasers and LEDs work, you have to get to know the atom’s tiny light switch: the electron energy level. Imagine an atom as a tiny building with a staircase. Electrons, the building’s residents, aren’t allowed to exist just anywhere. They can only occupy specific steps on the staircase, which we call energy levels. They can never be found in the space between steps. This is the quantum part of quantum mechanics—everything is quantized, or broken down into discrete packets, with no “in-between” states.
When an electron absorbs energy—from an electrical current, for example—it gets “excited” and jumps to a higher step. But like a person who’s just run up a flight of stairs, the electron doesn’t stay there for long. It wants to go back to a more stable, lower energy level. When it makes this jump back down, it must release the extra energy it absorbed. It does this by spitting out a tiny packet of light called a photon. The color of the light released is determined precisely by the size of the jump. A big jump creates a high-energy photon (like blue light), while a smaller jump creates a low-energy photon (like red light).
How LEDs Work: The Simple, Spontaneous Jump
An LED (Light-Emitting Diode) is a clever semiconductor device designed to make billions of these atomic light switches flip at once. At its core, an LED is made from two different types of semiconductor material joined together to form what’s called a p-n junction. When you apply voltage to the LED, you push electrons into one side and “holes” (the absence of an electron, which acts like a positive charge) into the other.
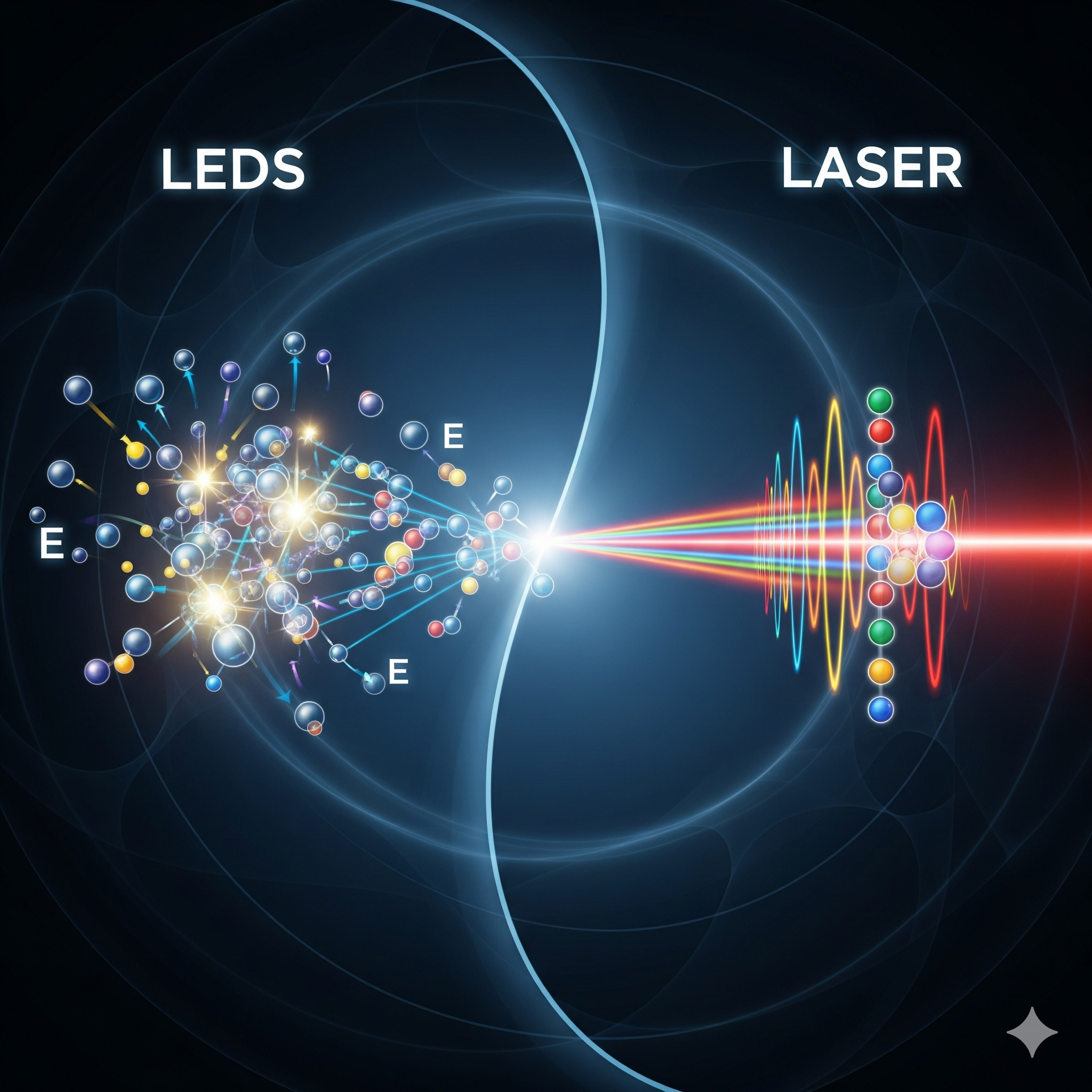
These electrons and holes meet at the junction and recombine. In this process, the electrons drop down to a lower energy level, releasing a photon of light. This process is called spontaneous emission because each electron jump happens on its own, with no coordination from the others.
Think of it like a giant crowd of people at a party, each one deciding to dance and shine a tiny flashlight at random. The light from an LED is the collective result of billions of these uncoordinated, spontaneous flashes. This is why LED light is non-directional and scattered, making it perfect for general lighting in homes, offices, and on your phone screen.
How Lasers Work: The Coordinated, Stimulated Jump
A laser (Light Amplification by Stimulated Emission of Radiation) takes the concept of spontaneous emission and supercharges it with a quantum trick. It relies on a special process called stimulated emission. A laser contains three key components:
- The Gain Medium: The material (solid, gas, or liquid) that contains the atoms that will produce the light.
- The Energy Source: A way to “pump” energy into the medium, forcing a large number of electrons to jump up to a high energy level (a condition called population inversion).
- The Optical Cavity: Two mirrors, placed on either side of the gain medium.
Here’s where the magic happens: instead of just waiting for electrons to spontaneously jump down, a laser “stimulates” them. An electron in a high energy level is hit by an incoming photon of just the right energy. This doesn’t absorb the photon; instead, it forces the electron to jump down right now, releasing a second, identical photon that is perfectly in sync with the first one.
This is a chain reaction. The two synchronized photons travel forward and hit two more excited atoms, which release four synchronized photons, then eight, and so on. The mirrors in the optical cavity reflect these photons back and forth through the gain medium, amplifying the beam to an incredible intensity. One of the mirrors is only partially reflective, allowing a small portion of the powerful, focused, and coherent (perfectly synchronized) beam to escape—this is the laser beam we know.
Conclusion
The difference between a simple LED and a powerful laser is a perfect example of how manipulating quantum mechanics can create radically different results. LEDs are a testament to spontaneous emission, a chaotic but effective way of generating light. Lasers are a masterclass in stimulated emission, which coordinates every photon for a single, powerful purpose. Both technologies showcase the fundamental truth that at the smallest scales, our universe operates on rules that are as bizarre as they are beautiful.